Do you have a budding scientist residing in your home? You're probably looking for ways to encourage their interest in science and STEM. Fortunately, even simple science experiments done at home can be highly educational and entertaining for children of all ages.
Cool science experiments and science activities for kids of all ages that you can do together on the weekend or after school. It's also a great way for them to learn about the scientific method, which they'll use throughout their education and possibly their entire lives!
Not all scientific experiments necessitate the use of expensive laboratory equipment or hazardous chemicals. There are numerous cool projects you can make with everyday household items. We've compiled a large collection of simple science experiments that anyone can try, and kids will love them!
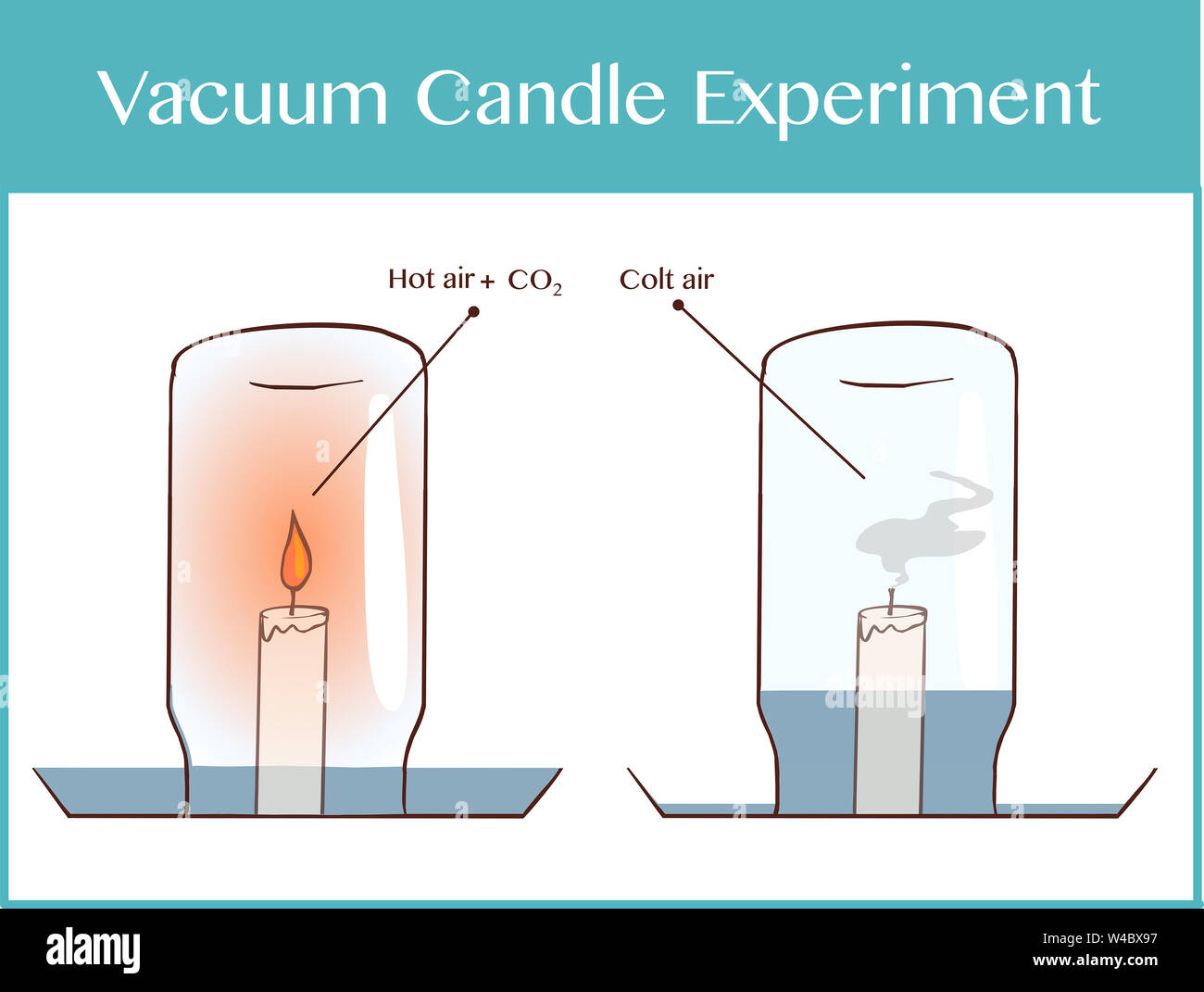
This post will teach you about Charles’s Law (Vacuum Candle Experiment) with this simple experiment. As the candle burns, using up oxygen and heating the air in the glass, the water rises as if by magic.
Science Experiment of Charles's Law - Vacuum Candle Experiment
Grade and Chapter Name:
Grade 5- Air
Grade 6- Air Around Us
Grade 8- Combustion and Flame
Things You Will Need:
1. Glass
2. Candle (shorter than the glass) and matches/lighter
3. Plate
4. Water mixed with Potassium permanganate/ink/food colour
Set-Up:
1. Fix the candle in the centre of the plate.
2. Pour the coloured water into the plate. (The colour will help you see the movement of water.)
3. Light the candle and invert a glass over the candle.
4. The candle extinguishes shortly after the glass is placed over it.
5. Soon, the water from the plate rises up into the glass. Why does this happen?
Observe:
The flame gets extinguished soon after the glass is covered over the candle.
The water gushes in soon after the flame extinguishes.
Concept:
The candle flame uses the Oxygen present in the air for combustion. When covered with glass, only Oxygen inside the glass is available for combustion. Once all the Oxygen inside the glass is consumed, the flame extinguishes. When the flame is glowing, the air around the flame expands due to the heat from the flame.
Once the flame is extinguished, the temperature inside the glass goes down and the expanded air inside the glass contracts, occupying lesser space than before. This creates a low pressure inside the glass.
Outside the glass, the normal atmospheric pressure is higher as compared to the low pressure inside the glass. This low pressure pulls the water from outside, causing the water to gush in from underneath the glass into it.
Watch the video - Vacuum Candle Experiment
Related Topics:
What is required for combustion- fuel, temperature, Oxygen. Products of combustion.
Try This:
Repeat the experiment using 2 or 3 candles and a wider glass. Do the candles burn for a longer or shorter duration? Wrap your palms around the glass. Does the glass get cooler once the flame extinguishes? Why?
$ads={2}
Credits: India Literacy Project (ILP)
You May Also Like 👇
Loading...
Tags
Science Experiments